

2024-11-05
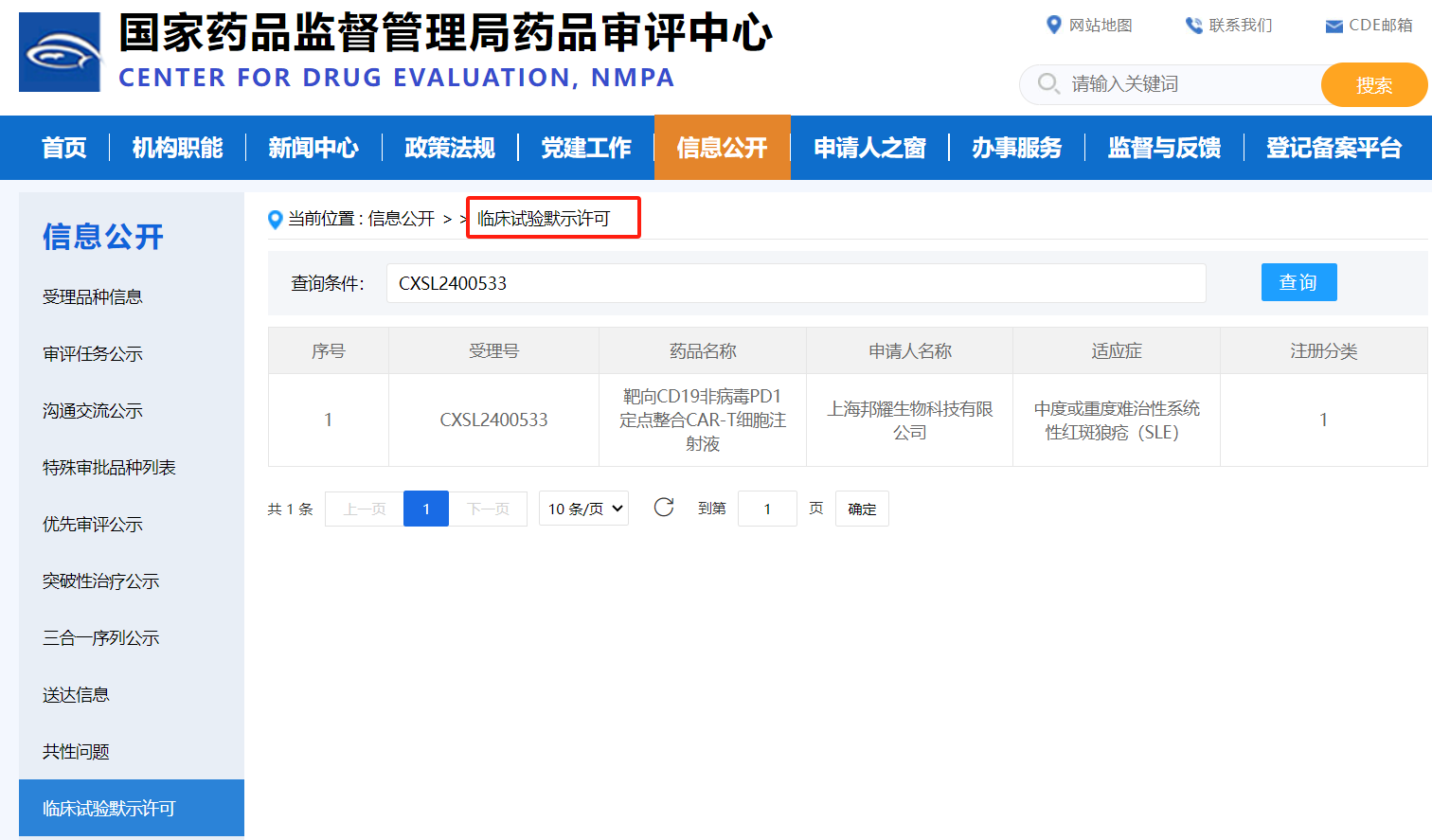
On November 5, 2024, BRL Medicine Inc. (hereinafter referred to as "BRL Medicine"), which focuses on gene and cell therapy, announced that its clinical trial application (IND) named "Targeting CD19 Non viral PD1 Targeted Integration CAR-T Cell Injection" (pipeline code: BRL-203), developed on the basis of the non viral targeted integration CAR-T platform with independent intellectual property rights, was officially approved by the Drug Evaluation Center (CDE) of the State Drug Administration of China. The indication for this IND is "moderate or severe refractory systemic lupus erythematosus (SLE)", which is an innovative CAR-T product developed by Bangyao Biotechnology in the field of treating autoimmune diseases. It is also the world's first non viral targeted integrated PD1-CAR-T product for treating autoimmune diseases and approved for IND, providing diversified options for the treatment of autoimmune diseases. It is worth mentioning that BRL Medicine non viral fixed-point integrated PD1-CAR-T therapy (pipeline code: BRL-201) has previously demonstrated excellent clinical safety and effectiveness in the treatment of recurrent or refractory B cell non Hodgkin's lymphoma (r/r NHL), and relevant research results were published in the international top academic journal Nature.
Autoimmune diseases are a heterogeneous group of diseases, with a common disease basis of impaired immune tolerance. Currently, immunosuppressants are commonly used in clinical practice to treat autoimmune diseases, but they have long treatment cycles, are prone to recurrence, increase the risk of infection and tumors, and some patients may not tolerate or have poor therapeutic effects. Targeted B cell monoclonal antibodies provide a new treatment option, but there are still issues such as relapse, infection, and poor efficacy in some patients after discontinuation. Given the important role of B cells in autoimmune diseases, many international research teams have attempted to apply CAR-T therapy to autoimmune diseases, including systemic lupus erythematosus, idiopathic inflammatory myopathy, systemic sclerosis, and myasthenia gravis, which have shown excellent clinical effects.
BRL Medicine non viral fixed-point integration PD1-CAR-T is safer, more effective and more accessible in the treatment of autoimmune diseases!
However, whether targeting tumors or indications for autoimmune diseases, products that have been launched or are currently under development still focus on autologous CAR-T products prepared using viral vectors, which inevitably pose potential risks of tumor formation, long preparation time and low accessibility, as well as high production costs and challenges. In contrast, the targeted CD19 non viral PD1-CAR-T product "BRL-203" developed by BRL Medicine using the non viral fixed-point integration CAR-T platform (Quikin CART) with independent intellectual property rights can solve the pain points of existing viral CAR-T, showing a huge advantage: safer, more effective, and more accessible.
Product advantages:
1. Fast preparation, low cost, and more accessible to patients
BRL-203 utilizes CRISPR/Cas9 gene editing technology, which won the Nobel Prize in 2020, to precisely edit the PD1 site in T lymphocytes without using a viral vector. It inserts CAR molecules targeting CD19 at specific sites, greatly reducing the high costs associated with using viral vectors in the production process. Moreover, the preparation time of traditional CAR-T products is relatively long, greatly increasing the waiting time for patients to take medication. The BRL-203 product adopts a non viral preparation method, with a simple process and only one step of preparation. It can simultaneously achieve sustained expression of CAR-T and regulation of endogenous genes in T cells, greatly reducing the preparation time of CAR-T, which can be completed in as short as 3 days, greatly improving production capacity and can be used for timely treatment of patients.
2. Ensure product uniformity for safer clinical use
Non viral targeted integration technology allows each CAR sequence to be precisely inserted into a specific site in the genome, avoiding the risk of tumor formation caused by random insertion and maximizing the safety and effectiveness of CAR-T products. In the previous clinical trial of BRL-201 treatment for relapsed/refractory non Hodgkin lymphoma using this innovative technology, 21 patients received treatment and no CAR-T related neurotoxicity or cytokine storm of grade 2 or above was observed, demonstrating the excellent clinical safety of non viral site integration PD1-CAR-T therapy.
3. Breaking the shackles of recurrence and difficult treatment for more effective treatment
In addition, existing clinical studies on BRL-201 have shown that the objective response rate (ORR) of patients is as high as 100%, the complete response rate (CR) is 85.7%, and the long-term benefits for patients are significantly higher than those of existing similar virus CAR-T products. As of February 10, 2024, the median overall survival (mOS) reached 39 months, and the median progression free survival (mPFS) reached 20 months, which is the best clinical result in high response rate and low toxicity of CAR-T cell therapy for refractory recurrent lymphoma worldwide. The single-cell sequencing results indicate that there is a high proportion of memory T cells and stronger anti-tumor immune function in BRL-201 cell products, and the CAR-T cells after reinfusion have the ability to persist for a long time. It can be seen that the non viral targeted integration PD1-CAR-T therapy has demonstrated excellent clinical effectiveness and will also demonstrate long-term benefits in the treatment of autoimmune diseases, benefiting patients.
At present, no other non viral targeted integration CAR-T products have been applied for and subjected to human clinical trials for autoimmune diseases in China. In the future, BRL Medicine will accelerate the development process of BRL-203 product, and is committed to bringing patients another drug choice with advantages in safety, effectiveness and cost.